You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
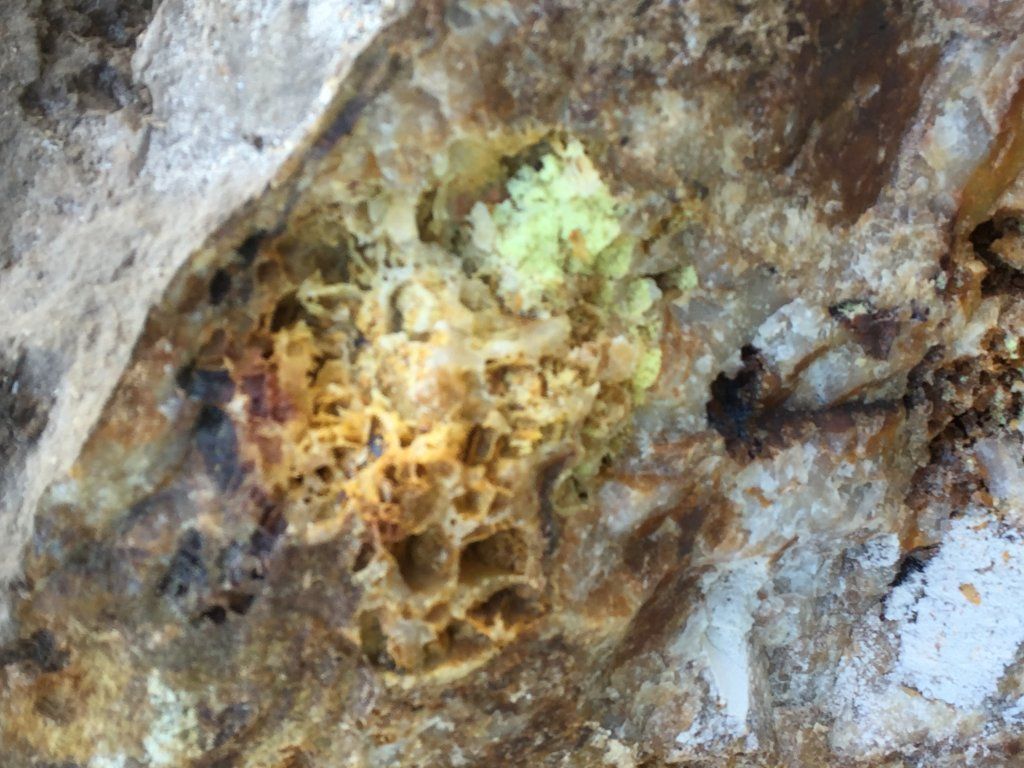
Any 1 know what the yellow sulphur looking stuff is
- Thread starter Bizmark
- Start date

Help Support Prospecting Australia:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
Yes - even though freshly cracked open, the sulphide minerals that used to be there have weathered out leaving the cavities.Bizmark said:Thats freshly cracked open the light green stuff is a mystery to me
Problem with yellow earthy looking minerals is that there are so many. Native sulphur is one, but it is relatively rare in most hard-rock mining fields in Australia. However many of the oxide minerals of elements like bismuth, molybdenite, antimony, tungsten, iron, uranium can be yellow (the "ochre" minerals). They are often soft and powdery with similar properties, and can be best separated only with a flame test.
I have found mineral similar to what you have in the photos in cavities in quartz. some lemon yellow waxy material was heavy enough to be right on the bottom of the pan mixed with the gold. I assumed that it was some form of Bismuth sulphide. The Green is maybe a Decomposition product of an Arsenic Cadmium Sulphide. Is there any gold in your sample?
Bismuth sulphide is not yellow and waxy, but more silvery and metallic:jethro said:I have found mineral similar to what you have in the photos in cavities in quartz. some lemon yellow waxy material was heavy enough to be right on the bottom of the pan mixed with the gold. I assumed that it was some form of Bismuth sulphide. The Green is maybe a Decomposition product of an Arsenic Cadmium Sulphide. Is there any gold in your sample?
http://webmineral.com/specimens/picshow.php?id=143&target=Bismuthinite#.XGk5__ZuJPY
I am not aware of there being an arsenic cadmium sulphide.
Sorry my bad I should have refreshed my memory before posting could it be this OXIDE of bismuth antimony and Iron. http://webmineral.com/data/Bismutostibiconite.shtml#.XGnBybhxXIU.
It could be so many things. Without a flame test or chemical analysis it is hard to pin down. Often remnants of the original sulphide that it is forming from is easier to identify, and the new mineral will contain metals that were in the old mineral.jethro said:Sorry my bad I should have refreshed my memory before posting could it be this OXIDE of bismuth antimony and Iron. http://webmineral.com/data/Bismutostibiconite.shtml#.XGnBybhxXIU.
I have a table of results expected, for blow pipe work drawn up for the Melbourne Technical Collage. It assumes that the user knows how to carry out blow pipe work but I would think that the required equipment is very hard to come by. Is there a modern technique that will give an indication of the elements present without the cost of expensive Xray Defraction machines. About the limit of my abilities is reducing Casiterite to metallic tin using Charcoal, Bycarb soda and a mapgas torch. Or oxidizing Arsenopyrite. under a flame (small piece with good ventilation, don't try this at home kids). :argh:
Do a search for Series on identifying minerals - part 7 FLAME AND BEAD TESTS under me (goldierocks)jethro said:I have a table of results expected, for blow pipe work drawn up for the Melbourne Technical Collage. It assumes that the user knows how to carry out blow pipe work but I would think that the required equipment is very hard to come by. Is there a modern technique that will give an indication of the elements present without the cost of expensive Xray Defraction machines. About the limit of my abilities is reducing Casiterite to metallic tin using Charcoal, Bycarb soda and a mapgas torch. Or oxidizing Arsenopyrite. under a flame (small piece with good ventilation, don't try this at home kids). :argh:
The requirements are simple. No doubt there are cheaper sources for platinum wire. We used to buy a short bit of wire and push it into a glass rod (as a handle) softened in a flame.
http://www.crscientific.com/platinumwire.html
http://www.crscientific.com/blowpipes.html
They also sell charcoal blocks.
How to use a blowpipe:
https://link.springer.com/referenceworkentry/10.1007/0-387-30844-X_11
Borax is readily available. However simply dropping powdered material in a candle flame can be useful.
https://www.stevespanglerscience.com/lab/experiments/flame-test/
Nichrome wire can be used as an alternative for platinum wire when testing for some elements (not all) - I would be wary with nickel and chromium when determining minerals containing those elements:
https://www.sciencecompany.com/Deluxe-Flame-Test-Chemical-Kit-With-Accessories-P16770.aspx
I imagine platinum-iridium wire would be as good as platinum wire, as it is very unreactive (better than nichrome wire I imagine):
https://www.labfriend.com.au/platinum-iridium-wire
On another note, those who have no testing tools for minerals might find this a good start - includes bar magnet, streak plate, hand lenses, acid bottle (for dilute hydrochloric acid - diluted 1:1 wirh water). For most hardness tests you only need your fingernail, a "gold" coin, a steel needle of tiny pocket-knife blade. and a piece of quartz.
https://www.homesciencetools.com/product/mineral-test-kit/
See my "Series on identifying minerals" on this site for more details.
Cheers Goldierocks Ive had a read through that, great resource for those of us interested in identifying metals, metaloids, and some of the other elements. Ill be getting a couple of blowpipes at least. and maybe some platinum coated nickel wire and an alchol burner, It'll give me something to do whilst sitting around the fire up at the hut near mitta, apart from whisky appeciation. :Y:
I have been outa serv for the wk working.. thanx for yur knowledge input.. this quartz Im smashing open has heeeaaps of different minerals going on... jus wondering is there a way I can get them tested or snd a sample of it.... I have brought home about 3 ton of rough quartz wit mass mineralization happening in it,, its from a vein of quartz about 4 ft wide 25 ft high from what I can tell..
Lots of fun minerals to find around Mitta. Cassiterite, bismuthinite, native bismuth, stibnite, gold, rare earth minerals, silver minerals - probably the best part of Victoria for variety.jethro said:Cheers Goldierocks Ive had a read through that, great resource for those of us interested in identifying metals, metaloids, and some of the other elements. Ill be getting a couple of blowpipes at least. and maybe some platinum coated nickel wire and an alchol burner, It'll give me something to do whilst sitting around the fire up at the hut near mitta, apart from whisky appeciation. :Y:
AaHa Im 45% sure its Unobtainiumnonspecficidium. An unstable isotope of Biggerbangernurinajam. Common Name is Toohottoholdium. :argh: Seriously though If its getting hot in your hand you shouldnt be holding it. Take it too your nearest University or Museum.  erfect:
erfect:
Similar threads
- Replies
- 0
- Views
- 168
- Replies
- 2
- Views
- 341